Proposal for a Brain Preservation Technology Prize
Ultimate goal: To spur research into the development of a surgical protocol that can reliably and demonstrably preserve a human brain’s precise neural circuitry for long-term (>100 years) storage. If such a procedure were available it would give interested persons a means of avoiding death and reaching the distant future.
Technology: Such a procedure would most likely involve either vascular perfusion of a person with cryoprotective agents and long-term storage at close to liquid nitrogen temperature, or vascular perfusion of a person with chemical fixative agents followed by a plasticizing agent and room temperature storage. Both techniques have already been successfully demonstrated on small pieces of brain, but advances in the chemical formulations, perfusion apparatus, and surgical technique are necessary to verifiably preserve all of the neural circuits in a human brain.
Given the already advanced state of the existing techniques, it is likely that preservation of a whole brain could be demonstrated within a 5 year timeframe if appropriate intellectual and monetary resources were devoted to the problem; unfortunately, a general lack of understanding of the potential for human brain preservation has severely curtailed such research. A Brain Preservation Technology Prize has the unique potential to break through this “social impasse” simply by its precise articulation of the criteria for success.
Prize structure: An invited group of independent scientists and medical doctors would create a set of requirements that a putative human brain preservation technique would have to demonstrate. Paramount to any such technique is the ability to demonstrate that the precise synaptic connectivity among neurons is preserved. This synaptic connectivity uniquely defines our memories, skills, and personality; in essence it defines our “soul”. A possible target milestone would require contestants to demonstrate their preservation technique on a large mammal (e.g. a pig), its brain would be stored for several months while being subjected to an accelerated aging environment (e.g. temperature swings), after which the brain would be dissected to look for gross structural damage, and, most crucially, ten or more tissue samples would be extracted from various brain regions and volume imaged using an electron microscope to verify intact neural circuits and synapses. Final judgment of results would rest with the original panel of independent scientists and medical doctors. If this set of expert-defined tests was successfully passed the contestant would win the prize.
Alternative Prize Ending: Instead of ending with the animal demonstration of the preservation technique, the contestant would be required to continue one step further to preserve the first human volunteer. This would be done in a grand ceremony where the brave volunteer embarks on the greatest adventure ever, stepping through a one-way door to the unknowns of the distant future. This would present a compelling human drama to highlight exactly what this Brain Preservation Technology Prize represents.
This addition is sure to raise the bar on what qualifies as a successful preservation demonstration in the animal model – the panel of scientists and medical doctors would now be certifying the technique for demonstration on a human. Of course, the comprehensive destructive testing performed to verify preservation in the animal model could not be performed on our human volunteer, but a host of non-destructive testing could be used to verify that the preservation worked to specifications.
There would likely be many volunteers, enough to choose one that is elder (perhaps diagnosed with a terminal illness to help assuage moral questions), famous, and justifiably suited to be our “human time capsule” emissary to the future. It is not unlikely that the donor for the prize purse may wish to take this honor (of being the first time traveler) for themselves.
Rationale: The idea of putting a person in “suspended animation” so they can reach future medical technology has been a staple of science fiction for decades. It has also been practiced, in an ad hoc way, by an eclectic group of amateur surgeons called cryonicists, which employ techniques whose efficacy is at best questionable.
Unfortunately, real quality research into effective brain preservation technology is almost nonexistent. Because of ineffective communication between the relevant disciplines (cryobiology, neuroscience, cognitive science, and nano-imaging) most scientists presume that such suspended animation is hopelessly beyond reach. In fact it is not. All that is required to make a form of suspended animation available today is a solution to a simple technical problem: “How does one preserve the synaptic connectivity of a human brain for long-term storage?”
It is long past time for this important idea to be reclaimed by mainstream science and medicine, and for serious research to be conducted to this end. The purpose of a Brain Preservation Technology Prize is to break through this “social impasse” by having an independent panel of scientists and medical doctors define a clear set of milestones which, if achieved, would warrant that such a preservation procedure be seriously considered as a viable medical alternative to death.
As I will describe below in the Background Information sections, I believe that such a prize could be won within the next five years, giving the thousands of aging space enthusiasts among us (and anyone that is optimistic about the future) an alternative to letting our dreams of seeing the distant future slip away.
Background Information
In 1962 Robert Ettinger put forward the radical idea that a person’s brain and body might be preserved using the best available techniques so that the person could reach future medical technology of sufficient advancement to restore health. This idea, because it is in principle scientifically sound, initially attracted much interest from both scientists and laypersons and gave rise to the practice of low-temperature (cryonic) preservation. However, even the best cryonic techniques of the 1960’s produced horrific damage to brain tissue as seen by light and electron microscopic examination. All reasonable scientists looking at this massive amount of damage to the neural connectivity of the brain rightly concluded that cryonic suspension using the existing techniques was hopeless. People selling cryonic suspensions to individuals were labeled as naive, or worse, quacks and charlatans, and the vast majority of scientists, especially cryobiologists, quickly distanced themselves from the entire enterprise.
This initial over-promise and then brutal rejection has left a tragic legacy; almost everyone has heard of cryonics but assumes the entire concept is fundamentally flawed. The concept is not flawed; it just requires diligent research and technology development to perfect the technique of brain preservation. Unfortunately, this research is not occurring. Only a handful of scientists are actively engaged in perfecting the technique of whole brain preservation today and the funding for such research is miniscule. This legacy of initial failures, widespread misunderstanding of the basic concept, ineffective communication among the relevant scientific disciplines (cryobiology, neuroscience, cognitive science, and nano-imaging), along with rampant superstition leading most laypersons to reject the basic scientific fact that the self resides in the physical brain, has formed an almost impenetrable “social impasse”. This social impasse has prevented intellectual and monetary resources from flowing to where they are needed to solve what is fundamentally a simple technical problem: “How does one preserve the synaptic connectivity of a human brain for long-term storage?”
All people that are optimistic about the future and would like to experience its wonders personally should be deeply troubled by this state of affairs. For anyone over the age of 30, brain preservation technology almost certainly represents, for example, their only hope to experience firsthand space travel beyond the earth-moon system. Most futurists believe that the inexorable degeneration of one’s health due to aging and disease will eventually be conquered, but only the most quixotic among us presume that such a plethora of medical miracles will be perfected in the next few decades.
Brain preservation technology, in contrast, is well within our reach today (as I detail below) and there is every reason to expect that it could be perfected to the level of a common surgical procedure in the next five years if the proper resources were allocated.
It should really be viewed as a form of “medical time travel” (Lemler et al. 2004) designed to keep our brain “alive” but unconscious until future medical techniques (e.g. regrowing organs and whole bodies, or brain scanning and uploading into robotic bodies) allow us to experience the riches of the world again. Such techniques may take hundreds of years to perfect, but as a preserved brain we can wait as long as is necessary.
I provide in this section a technical overview of the current state-of-the-art in cryopreservation, chemical preservation, and automated neural circuit tracing technology. I provide a more in-depth discussion of chemical preservation technology since I feel that it is potentially the best option for human brain preservation today. The reasons for this are: 1.) Chemical preservation allows for long-term storage at room temperature (thus avoiding the ever-constant risk of catastrophic destruction due to thawing). 2.) Chemical preservation allows for easier and more thorough quality assessment of brain preservation attempts. 3.) Detailed scenarios exist showing how today’s automated neural circuit tracing technology could, in the future, be scaled up and applied directly to a chemically fixed and plasticized brain block thus mapping all the neural connections within that preserved brain (Sandberg and Bostrom, 2008).
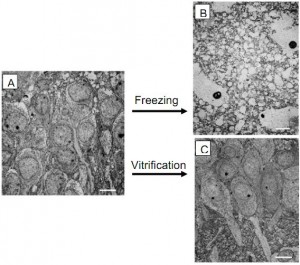
It is now 45 years since Ettinger’s original proposal and the technology for long- term structural preservation of brain tissue, despite the low funding levels, has advanced spectacularly in the interim. Recently Pichugin, Fahy, and Morin (2006) have demonstrated excellent structural preservation of half-millimeter thick brain slices and even recovery of function with simple rewarming. Figure 1A is a transmission electron micrograph from a rat hippocampal slice. Figure 1B shows the horrific damage (destroyed cells) that occurs when such a slice is “preserved” using a freezing technique typical of those employed early in cryonics. Such damage is clearly irreversible by any future technology and it should come as no surprise that such techniques were flatly rejected by the scientific and medical community. Figure 1C shows the current state-of- the-art in preserving brain slices using low-temperature vitrification instead of simple freezing. This slice has been brought down to a temperature of -130oC (a temperature so low that it could be stored for hundreds of years without change) and then rewarmed seemingly without damage. The delicate structure of the brain slice appears well preserved and in fact they report that it retains its basic functioning after rewarming. Fahy et al. (2004) have demonstrated similar techniques to preserve whole rabbit kidneys. It is important to understand, however, that these results have been obtained on small tissue volumes, the functional tests measured only peripheral aspects of neural function, and there has been no public report of such techniques applied to whole brains showing uniform preservation of structure across the whole brain down to the synaptic level. Typical problems reported when such techniques are applied to large tissue volumes are pockets of ice formation due to insufficient perfusion of cryoprotective agents, and large cracks due to thermal stress.
Figure 1. (A) Electron micrograph of a small region of a rat hippocampal slice. (B) Electron micrograph from such a slice that was “preserved” using a simple freezing technique. Extensive damage is evident. (C) Electron micrograph from such a slice that was preserved by vitrification and cooled to -130oC using state-of-the-art techniques.
This slice shows excellent structural preservation when compared to the control slice (A). Scale bars are 10 microns. Adapted from (Pichugin, Fahy, and Morin 2006).
Our fundamental understanding of how the brain functions and how in principle a preserved brain may be revived has also advanced spectacularly in the last 45 years. Low-temperature preservation techniques like the one described above strive for preservation not only of the structure of the brain but also its function in the sense that it could in principle be revived by simple rewarming and reperfusion with blood. But the requirement that function can be revived by simple rewarming is much too stringent in that it assumes no assistance from the advanced technologies of the future. We now understand that the true measure of success should be that a procedure preserves the structural connectivity of the neuronal circuits of the brain along with enough molecular level information necessary to infer the functional properties of the neurons and their synaptic connections. As long as that information is preserved, future technology can read it out and use it to construct a computer emulation of the brain’s functioning. This realization opens the door to other techniques of brain preservation not involving low temperature.
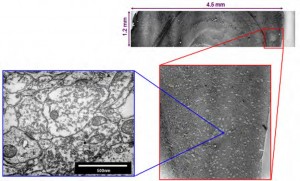
The figure below shows a thin section off the surface of a plastic block containing part of the cerebral cortex of a mouse. This is a scanning electron microscope (SEM) image. Successive images zoom in on this slice by a factor of 10,000x to reveal individual synapses in great detail. The structural connectivity of this small piece of brain tissue is perfectly preserved. How was this done?
Figure 2. SEM images, at progressive larger magnifications, of a 50nm thick section of chemically fixed and plastic embedded mouse cortex.
This block of brain tissue was preserved using a chemical preservation technique which has been in standard use by biological electron microscopists for decades. In this standard technique, a live mouse is first made unconscious by an injection of a strong anesthetic. Its chest is opened surgically and a large needle is inserted into the left ventricle of the heart. This needle is used to perfuse chemicals directly into the vasculature of the mouse. A hole is made in the heart’s right atrium to allow exit of blood and chemicals. First, a buffer solution is perfused in order to wash the blood out of the vasculature. This is followed by a fixative solution like glutaraldehyde which chemically crosslinks (i.e. ‘fixes’) all the proteins in every cell. The brain is then surgically removed and a vibrotome is used to produce 50-100 micron thick slices of the brain. These slices are immersed in a solution of osmium tetroxide which fixes all the lipids in every cell within the slice. These two steps (fixation of proteins and fixation of lipids) are crucial in that they use chemical bonding to in effect “glue” all the cellular structures together. This fixes the structure of the brain in place so that it can undergo the following steps leading to plasticization.
The fixed tissue slice is then dehydrated by immersing it in a series of graded alcohols, starting with a low concentration and going to a final solution of pure alcohol. This series of alcohols is designed to remove all the water from the tissue and replace it with alcohol which allows an organic solvent like propylene oxide to penetrate into the tissue.
The tissue slice is then put through a series of alcohol/ propylene oxide solutions starting with a low concentration of propylene oxide and going to a final solution of pure propylene oxide. This series is designed to remove the alcohol from the tissue and replace it with propylene oxide. Why propylene oxide? Because as an organic solvent it can dissolve plastic resin and allow the resin to penetrate deep into the tissue. It is important to understand that if the proteins and lipids within the brain slice had not been fixed previously (by the glutaraldehyde and osmium tetroxide) they themselves would be dissolved by this organic solvent resulting in complete disruption of the brain’s cellular structure.
The tissue is then put through a series of propylene oxide/ resin solutions starting with a low concentration of resin and going to a final solution of pure resin. This is designed to impregnate plastic resin into every cell in the tissue slice. Finally this resin- impregnated brain slice is put in a block mold, covered with additional resin, and cured in a 60o Celsius oven hardening it into a solid block of plastic.
The result is a hard plastic block containing a slice of brain tissue in which all the water has been removed from every nook and cranny of intra and extracellular space and has been replaced with hardened plastic. The structure of the neural circuits is perfectly preserved in this plastic matrix creating, in essence, a perfect fossil which preserves every synaptic connection in great detail.
Because the tissue is now rigidified inside this plastic block, it can be sliced very thinly. Typically, a diamond knife is used to slice ultrathin sections (just 50 nanometers in thickness) off the plastic block. These can be stained with heavy metals and viewed in an electron microscope at resolutions approaching 1nm to obtain the types of detailed images of individual synaptic connections shown above.
The electron microscope’s resolution is insufficient to image individual molecular structures like the proteins in ion channels and neurotransmitter receptors. These molecular-scale components may be important for determining the functional properties of individual neurons and synapses. Are these proteins lost in the chemical preservation process described above? No. These crucial proteins are fixed in the exact positions they were when the animal was first perfused with glutaraldehyde. In fact, even today immunolabeling techniques are often applied to such plasticized brain sections to allow ion channels and neurotransmitter receptor locations to be imaged in conjunction with electron microscopic investigations of synaptic structure.
In summary, the standard chemical fixation and plasticization technique used for decades in biological laboratories is capable of preserving both the structural and molecular information of neural circuits. If this technique could be applied to an entire human brain, the resulting plastic block could sit on a shelf for centuries without change, and would contain inside it sufficient information to allow future technology to upload the person’s mind into a computer emulation bringing him back to life.
Why then does this standard protocol not meet the criteria for a human brain preservation technique? The reason is diffusion. In the above procedure, just after the mouse was perfused with glutaraldehyde, the brain was surgically removed and sliced into 50-100micron thick sections using a vibrotome. This step is crucial in the above procedure because the osmium tetroxide fixation (along with the subsequent steps) is performed by simply immersing the tissue in solution, not by perfusing it. Osmium tetroxide can reliably diffuse into tissue only to a depth of a few hundred microns so the brain had to be sectioned into 50-100 micron thick vibrotome slices prior to this step. When larger volumes are attempted the inner regions of the volume contain severely disrupted neural tissue, only the superficial regions are well preserved.
Brain tissue that has been embedded in a plastic block can be sectioned without destroying neural connections, but when soft brain tissue is vibrotome sectioned (in the way it was prior to the osmium fixation step) the knife rips through the soft, watery neural circuitry leaving a path of utter destruction in its wake (spilled cellular contents, torn and distorted axons, etc.). The neural circuits deep inside these vibrotome slices are undamaged and can be perfectly preserved in plastic during subsequent steps, but neuronal processes that lie within a few microns of the top and bottom cut surfaces of these vibrotome slices are utterly ruined destroying any possibility of tracing neural circuits that span multiple vibrotome slices.
Toward a whole brain chemical preservation protocol:
Is it possible to modify the standard fixation and plasticization protocol to allow an entire human brain to be preserved all at once? The answer is almost certainly yes, and I believe a concerted effort could achieve this goal in less than five years. The key to accomplishing this is to perform most or all of the processing steps directly via perfusion through the vasculature. The capillaries of the vascular system come within a few tens of microns of every part of the brain so slow diffusibility through the tissue would no longer be a problem. Perfusion, unlike immersion, is insensitive to the volume of the tissue being preserved, so the entire brain (of a mouse or a human) could be preserved at the ultrastructure level using such a technique.
Vascular perfusion itself is routinely used. As noted above, vascular perfusion with glutaraldehyde is the first step in the current tissue preparation process. Perfusion fixation with osmium tetroxide has been demonstrated on whole brains (Palay et al. 1962) (Fig. 3) but was followed by dissection into tiny pieces suitable for immersion dehydration and embedding. As noted previously, such gross dissection (before resin infiltration and curing) destroys the possibility of tracing fine circuits across dissection boundaries.
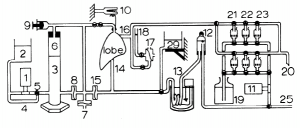
In lung, serial vascular perfusion first with glutaraldehyde, followed by osmium tetroxide, then followed by uranyl acetate has shown excellent preservation results (Bachofen et al. 1982). Even the step of dehydration has been demonstrated via vascular perfusion (in lung) meaning that glutaraldehyde, osmium tetroxide, uranyl acetate, and several alcohol concentrations were successfully perfused through the same lung in series leading to excellent, large-volume preservation (Oldmixon et al. 1985). The complicated apparatus that accomplished this is shown in figure 3. To my knowledge, such serial perfusion has never been attempted in brain, but it seems achievable given its success in lung.
Figure 3. (Left) Electron micrograph of a capillary and surrounding neuropil in the cerebellar cortex of a rat whose entire brain was fixed via vascular perfusion with osmium tetroxide (from Palay et al. 1962). (Right) Schematic of serial perfusion apparatus used for lung tissue preparation (from Oldmixon et al. 1985).
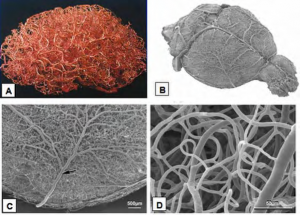
The journal papers referenced above demonstrate that the vasculature remains open and intact even after glutaraldehyde and osmium fixation (the papers checked for edema and for uniformity of preservation). This means that low viscosity fluids (e.g. alcohol) can continue to be perfused through the tissue with impunity. However, plastic resin solutions typically have much higher viscosity. Can the vascular system be perfused with resin down to the level of the smallest capillaries? In fact it can. The technique of “vascular corrosion casting” is used routinely to study the 3D structure of the vascular system. It involves perfusing the animal’s vasculature with resin, letting it cure, and then dissolving the surrounding tissue with a strong base. Fig. 4A shows a vascular corrosion cast of an entire human brain. Fig. 4B shows a vascular corrosion cast of an entire mouse brain, and figs. 4C and D zoom up on this mouse brain using a SEM to demonstrate that even the finest capillaries have been successfully filled and reproduced with this technique.
Figure 4. (A) Vascular corrosion cast of an entire human brain. (B-D) Vascular corrosion cast of a mouse brain from (Krucker et al. 2006).
The plastic used in these vascular corrosion casts is different from that used in preparation of tissue for tissue preservation. Its viscosity is controlled over a short period of time by dissolving it in a set amount of solvent (Krucker et al. 2006) and it can be cured quickly since there is no need in a vascular corrosion cast for the resin to infiltrate the tissue surrounding the blood vessels. New protocols for the timing, solvents, concentrations, etc. would need to be developed to allow resin infiltration via perfusion, but these results suggest that such a technique is possible.
In summary, the standard fixation and plasticization protocol only allows small tissue volumes to be preserved. By switching the standard protocol’s immersion steps to perfusion, an equivalent quality of tissue preservation should be achievable for volumes as large as an entire human brain.
Importance of performing procedure before death:
It is important to understand that the standard fixation and plasticization protocol is started while the animal is still alive. If the animal’s heart is allowed to stop for even a few minutes before the glutaraldehyde is perfused into the vasculature, then the quality of the preservation is markedly reduced. This fact will also be true for any whole brain protocol based on perfusion.
The protocol depends on a functioning vasculature to carry the chemicals throughout the brain, and the vasculature’s function begins to be compromised almost immediately if oxygenated circulation stops. If, due to vasculature problems, fixation chemicals are prevented from reaching some areas of the brain, then the proteins and lipids in those areas will be disrupted in subsequent process steps. If alcohol and solvent is unable to reach areas, then those areas will not be sufficiently plasticized leading to disruption of the neural circuitry within. Many other factors can also interfere with preservation including vasculature problems caused by the health state of the animal. The development of a surgical protocol for human brain preservation will need to take into account all of these factors.
Neural circuit imaging technology:
Until quite recently it was impossible to trace large volumes of neural circuitry at the electron microscope level. Blocks of plasticized brain tissue would be sectioned at 50nm and collected in a manual, error-prone process for imaging in a transmission electron microscope. Even with heroic efforts, this manual process could only handle tiny volumes smaller than 0.01mm3. However, over the last five years three new automated imaging techniques have been introduced that stand to revolutionize the tracing of neural circuitry over large volumes, and that for the first time represent real precursors for the types of future technologies necessary to revive a preserved brain. All of these techniques are based on imaging chemically preserved, plasticized tissue in a scanning electron microscope (SEM). These techniques are diagramed in Figure 5 below.
Insert image here
Figure 5. New high-throughput techniques for electron-microscopic volume imaging of neural circuits.
The Serial Block Face Scanning Electron Microscope (SBFSEM) (Denk and Horstmann 2004) works by mounting the plastic block of brain tissue in the vacuum chamber of a SEM. A diamond knife within the chamber scrapes successive 30nm layers off the top surface of the block. Between scrapings, the SEM’s electron beam is scanned across the face of the block and electrons backscattered off of heavy metal staining atoms in the tissue are read off as a signal to produce an image of the block face. This interleaved scraping and imaging continues to produce a three dimensional image of the neural circuitry within the block.
The Focused Ion Beam Scanning Electron Microscope (FIBSEM) (Knott et al. 2008) works on a similar principle except, instead of using a diamond knife, a focused beam of gallium ions is used to ablate as little as 15nm of material off the surface of the block. The Automatic Tape-collecting Lathe Ultramicrotome (ATLUM) (Hayworth et al. 2006, Hayworth 2007) is a device that uses a diamond knife to section a plastic tissue block into thousands 30-50nm thick sections. Each of these ultrathin sections is automatically collected within the machine on a long pickup tape. This tape is stained with heavy metals, cut into smaller strips, and the strips are adhered to a set of metal plates creating an Ultrathin Section Library (UTSL) of the original brain volume. UTSL plates can be loaded into the SEM for electron backscatter imaging at a resolution of 5nm. The plates can also be tilted relative to the SEM’s electron beam allowing the depth information within a single ultrathin section to be explored (this allows the eventual volume image of the brain block to attain finer resolution than the section thickness itself). Finally, sections in a UTSL can be immunostained to reveal the location and concentrations of molecular-scale components like ion channels and neurotransmitter receptors.
These scanning techniques provide the necessary tools to verify the quality of current preservation attempts, and straightforward scaling up of these techniques provides a roadmap to future reanimation of a preserved person by uploading the scanned neural circuitry into a computer emulation controlling a robotic body (Sandberg and Bostrom, 2008).
The key to breaking through the social impasse: Education
The most fundamental stumbling block to progress on brain preservation (and to its eventual application as a medical procedure) is not a technical one, it is the “social impasse” mentioned above. The fundamental scientific knowledge and technology exists today to perfect brain preservation and raise it to the level of a common surgical procedure performed routinely in hospitals. Unfortunately, very few people (scientists and laypeople alike) understand that the key to beating personal death lies in developing a technique that preserves, for long-term storage, the synaptic connectivity of their brain. Fewer still realize that such a technique is well within our current technological grasp. In order to see this potential, a person must understand and agree with the following six assertions, all of which are well founded in science:
Assertion #1 Soul = brain: The past hundred years of research in cognitive science and neuroscience have firmly established that the entirety of what we call our mind, or our soul if you like, is in actuality a complex information processing stream computed by the neuronal circuits in our brain. Our subjective first-person conscious experience is synonymous with the particular symbolic and subsymbolic computations performed by the moment-to-moment firing patterns of our neurons. This scientific fact is about as well established as the fact that we evolved from a common ancestor with the chimpanzee or the fact that the earth is billions of years old. Popular culture may continue to struggle with these revelations from science on the nature of the human condition but those learned in the relevant scientific fields no longer do.
Assertion #2 Memories = structure: The memories and computational processes that define ‘you’ are uniquely defined by the structure of your brain with only the last few moments of memories dependent on the brain’s dynamic electrical and chemical activity. One of the ways we know this is that the surgical procedure of profound hypothermia and circulatory arrest shuts down all neural activity in a person for almost an hour with no long-term detrimental effect (Sullivan et al. 1999). ‘You’ are written into your brain’s structure at the level of the synaptic connections between the neurons.
Assertion #3 Structure can be preserved indefinitely: Two technologies (vitrification and chemical fixation) exist today that have demonstrated the ability to preserve the precise synaptic structure of small pieces of brain tissue over indefinite periods of time. As detailed in the technology sections above, long-term preservation of the precise synaptic connectivity of an entire human brain (via perfusion) using these technologies is clearly feasible in principle, and a sober appraisal of the current state of the art puts such an accomplishment within a time frame of 5 years if sufficient research effort were devoted to the task.
Assertion #4 Structure can be scanned into a computer: Electron microscopic imaging technologies (SBFSEM, FIBSEM, ATLUM) exist today that have demonstrated the ability to extract the precise synaptic connectivity patterns from small blocks of preserved brain tissue. There is a straightforward path for these technologies to be scaled up to the level of a whole human brain. Although the technology for routine scanning of entire human brains will most likely require many decades of further research to make practical, a brain preserved today can wait as long as necessary for such research to succeed.
Assertion #5 A brain can be emulated in a computer: The science of neuronal circuit emulation (simulating a neuronal circuit’s functioning in a computer) is already quite advanced (e.g. Markram 2006) and there is every reason to expect that within this century humanity will be able to emulate the original functioning of a preserved brain given the map of synaptic connectivity provided by electron microscopic scanning technologies like those described above (Kurzweil 2006)(Sandberg and Bostrom, 2008).
Assertion #6 Our emulated brain is us (corollary of #1): The computer emulation of a preserved and scanned brain would perform the same symbolic and subsymbolic computations that existed in the original biological brain, and therefore this emulation would be the original person in every way that is important to that individual. It will be trivial for future technology to create a sensitive, dexterous and supple robotic body (better in every way than the original biological shell) for such an emulation to control and experience the world through.
A Brain Preservation Technology Prize is uniquely suited to the task of raising the level of intellectual discourse around these issues, and to educating the public about the basic science underlying them and about the current state-of-the-art of preservation, scanning, and computer emulation technology. If one understands and accepts the previous assertions then one is inexorably drawn to the conclusion that the technology to reach the distant future is within our grasp. It merely requires the ability to preserve the structure of a human brain for long-term storage. Such technology is already well advanced and could be demonstrated in the next 5 years. We merely need to invest the proper monetary and intellectual capital.
If you have ever gone to a time capsule internment ceremony, e.g. as part of the dedication ceremony of a building, you realize just how interesting an experience it is. It causes you to step outside the day-to-day concerns of your life and draws you to imagine the people one hundred years hence that will be opening the time capsule. What will their world be like? Will they have cured aging and disease? Will they have solved the inexorable problems of our time: poverty, warfare, prejudice, greed? Will they retain our adventurous sprit and have developed technology that lets this sprit freely roam throughout the galaxy? Will they have developed satisfying answers to our deepest scientific questions concerning physics, cosmology, and consciousness? Will they have expanded the human potential in ways we cannot even imagine?
You look down at the collection of photographs, documents, and heirlooms the designers of the time capsule have thought fit to include in order to convey to the people of the future what life was like today. You find yourself envying those simple scraps of paper. They will “see” the future but we will not.
The perfection of brain preservation technology will allow us to hitch a ride to the future in such a time capsule. The technology is within reach, and a Brain Preservation Technology Prize is uniquely suited to educate the world about this possibility and uniquely suited to help mobilize the intellectual and monetary resources needed to achieve it.
- Login to post comments