Glutaraldehyde and reduction techniques for immunolabeling
This blog post, by intrepid BPF volunteer Benjamin Bechand, addresses the question of immunolabeling molecules following glutaraldehyde preservation of a tissue. This is a chemistry topic that is highly relevant to many questions in the field of brain preservation.
Glutaraldehyde is a powerful preservation agent used often in microscopy as it allows for highly retained cellular structures. However using it does provide some problems when it’s time to observe these structures under an electron microscope.
Glutaraldehyde (CH₂(CH₂CHO)₂) is a 5 carbon chain with an 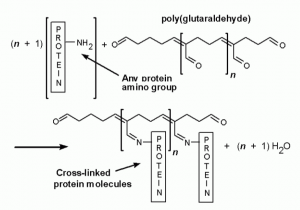 aldehyde functional group on each end. Like its cousin formaldehyde, the aldehyde functional group allows it to act as a biological preservative. When exposed to a cell, each aldehyde will covalently bind to different structures with amine groups. Think of this as a long rope with two sticky ends. The ends will connect to various things in the cell creating a long chain. With enough glutaraldehyde a web-like tangled mess will form inside the cell, trapping all the structures in place, and killing the cell.
aldehyde functional group on each end. Like its cousin formaldehyde, the aldehyde functional group allows it to act as a biological preservative. When exposed to a cell, each aldehyde will covalently bind to different structures with amine groups. Think of this as a long rope with two sticky ends. The ends will connect to various things in the cell creating a long chain. With enough glutaraldehyde a web-like tangled mess will form inside the cell, trapping all the structures in place, and killing the cell.
This is a really useful method of preservation because it traps everything in place giving an accurate picture of the cell while it was alive. Additionally it leaves proteins intact and able to be studied.
The problems begin at the next stage when we want to observe our preserved cell. Immunolabeling is a technique where biospecific antibodies are attached to florescent tags that glow under certain wavelengths of light. You can flush cells with immunolables to observe the location and concentrations of molecules (like proteins) with high accuracy. However, in your glutaraldehyde preserved cell there will be sticky aldehyde ends in abundance that will readily bind to your antibodies to give auto- florescent (washed out) images. So before using immunolabeling we much first resolve the unreacted aldehyde groups in our cell.
Glutaraldehyde reduction
In order to prevent glutaraldehyde from trapping our immunolabels we must inert the aldehyde groups by reducing them to alcohol functional groups. This is easily done with a reducing agent such as sodium borohydride or Schiff’s reagent.
Schiff’s Wash
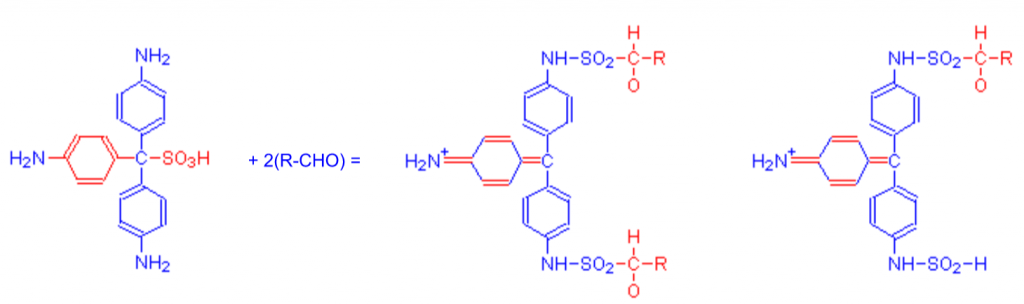
Schiff’s reagent is pararosanilin in a sulfurous acid solution. This reagent was originally developed to detect the presence of aldehydes. When Schiff’s reagent binds to an aldehyde group a disruption occurs in the chromophore and yields an easily detectable red color.
There are many different ways to prepare the sulfurous solution, so here are a couple of methods used in labs specifically for the purposes of neural immunolabeling:
- Immunofluorescence and glutaraldehyde fixation. A new procedure based on the Schiff-quenching method. Journal of Neuroscience Methods, Volume 77, Issue 2, Pages 191-197 (PubMed).
- Neuron-derived D-Serine Release Provides a Novel Means to Activate N-Methyl-D-aspartate Receptors. May 19, 2006 The Journal of Biological Chemistry, 281, 14151-14162 (PubMed).
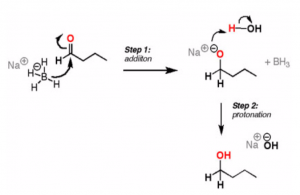
Sodium Borohydride is another commonly used reagent. It is more standardized in its application.
 One lab used an interesting method in the paper shown below. After treatment with sodium borohydride, they then exposed to sample to non-specific antibodies not attached to a florescent tag. Any unreduced aldehydes then bound to the non-florescent antibody allowing for secondary treatment with the intended immunolabel.
One lab used an interesting method in the paper shown below. After treatment with sodium borohydride, they then exposed to sample to non-specific antibodies not attached to a florescent tag. Any unreduced aldehydes then bound to the non-florescent antibody allowing for secondary treatment with the intended immunolabel.
Cytoplasmic microtubular images in glutaraldehyde-fixed tissue culture cells by electron microscopy and by immunofluorescence microscopy. PNAS. vol. 75 no. 4, 1820-1824 (PubMed).
Now that you have a well preserved, aldehyde free sample you are all ready to immunolabel!