我们需要非低温性的脑保奖吗?
Note: The following is my personal opinion, and does not necessarily reflect the views of my colleagues at BPF. I am a futurist, not a neuroscientist. Any mistakes here are my own. Please point out any you find in the comments, so I can revise as necessary, thanks.
Cryonics, Old and New
In 2016, Motherboard made a lovely 23-minute video, Frozen Faith: Cryonics and the Quest to Cheat Death. I recommend it for those new to the topic of brain preservation. The video does a great job showing the early inspiration for cryonics (low temperature storage of human beings), beginning in science fiction in the 1930s. It also charitably portrays traditional cryonicists like Max More, PhD, of Alcor, and earnest new cryonicists like Jordan Sparks, DMD, of Oregon Cryonics. But most importantly, in my view, it describes the grave limitations of traditional cryonics, which seeks to preserve individuals using a process called vitrification (perfusion with antifreeze) for later reanimation in biology, presumably via advanced AI and nanotechnology. Unfortunately, as the cryobiologist Mehmet Toner, PhD of Mass General Hospital seeks to demonstrate in the video, vitrification works for single cells but has never worked reliably for large tissues, despite decades of efforts in organ cryopreservation. Vitrification can minimize ice crystal formation, but other problems, including osmotic dehydration, and ischemic damage during long perfusion times, have greatly limited the use of cryopreservation for large tissues. Vitrified connectomes don’t look normal under electron microscopy, and cryonicists haven’t figured out a way to make them look normal. That may be one reason cryonics has never been able to gain support, in the sixty-odd years of its existence, from any group of mavericks in either the cryobiology or neuroscience communities.
Fortunately, there are now a promising group of new cryonicists, exemplified by Robert McIntyre at Nectome. McIntyre was the primary researcher on a team that won our small and large mammal Brain Preservation Prizes in 2016 and 2018. These new cryonicists don’t want to preserve brains for future reanimation in biology, but for automated scanning and uploading, into digital brain banks, and software “emulation” of brain processes. They aren’t perfusing brains with antifreeze substances to preserve them, but rather with chemicals like acrolein and glutaraldehyde, which very rapidly “fix” the structural and molecular features of our connectome and synaptome via chemical crosslinks, and then further stabilize that fixation by putting the brain in extreme cold storage. This “cryofixation” process apparently even preserves molecular tags (phosphorylation, etc.) on neural proteins, the smallest features of our brains that some have implicated in learning and memory. All of these features, once preserved, can presumably later be scanned and uploaded into digital emulations, once the science and technology develops. Indeed, it is rapidly developing now, in neuroscience labs all around the world.
In 2016, I and two of my BPF colleagues, Michael Cerullo, MD, and Keith Wiley, PhD covered both camps of cryonicists, the Reanimators 和 Uploaders, and a third camp, the Uncertains, in a brief article, The Three Camps of Brain Preservation. In the four years since, a handful of neuroscientists are now willing to state publicly, and several more privately, that they think the process of “aldehyde stabilized cryopreservation” (ASC), that won our Prizes may be sufficient to retrieve meaningful neural information, specifically long-term memories, once science has fully cracked the neural codes for memory storage. Recently, the Nectome team has been investigating how ASC preserves simple memories in simple animals, beginning with nematodes. See Sharon Begley’s Stat News article, Jan 2019 for an overview of their important work.
One of several aims of BPF’s 神经科学进取奖 is to evaluate advances in our understanding of the encoding of learning and memory. That evaluation won’t be easy, which is why annual awards in this prize are planned to continue over ten years. We gave out our first set of awards to four very deserving teams at a gathering adjacent to the Society for Neuroscience meeting last year, and we have nine years of awards still ahead of us. Thankfully, the philanthropist who funded these prizes had the vision to take a long-term view.
Noncryogenic Fixation, a Neglected Class of Room-Temperature Preservation (RTP) Processes
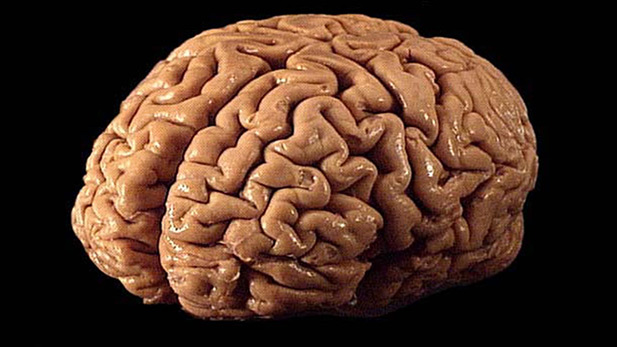
A plastinated brain (Photo: Allan Ajifo, CC BY 2.0)
When we take that long-term view, I would like to point out there are a wide variety of noncryogenic chemical fixation processes that will prevent cells from enzymatically degrading their cellular, synaptic, and molecular structures after death (that process is called autolysis), and which presently appear to keep critical neural structures stable indefinitely at room temperature.
One form of (apparently) low-fidelity fixation many of us are familiar with is called plastination. Brains are commonly plastinated for educational purposes today, as in the specimen at right. This process uses uses heat and solvents to evaporate the brain’s water and dissolve and extract the brain’s fats, and replace these voids with various types of plastics. Current plastination techniques grossly preserve brain structure but not its fine neural detail (Correction: Andy McKenzie points out in the comments that there are plastination techniques that do retain neural ultrastructure–much more work is needed to determine how well features like the synaptome might be preserved).
Another form of high-fidelity fixation has been used by neuroscientists to preserve and examine the fine structure of neural tissue since the advent of electron microsopy in the 1930s. As with cryofixation, this type typically begins by first diffusing or perfusing in an aldehyde or other small molecule that will form crosslinks between all the tissue’s proteins, locking everything down so it can’t change after death. Then additional fixation molecules, like osmium tetroxide, are added to stabilize the fats in their place, rather than washing them out, as occurs in plastination. Finally, solvents are used to gently push out water and push in an epoxy polymer (resin). One commonly used resin is called Epon. This more elaborate form of fixation is called plastic embedding (note the close similarity of this phrase to plastination). Alternatively it might be called “resination”. There are a great variety of techniques and substances, including resins and waxes, used to stabilize tissue at room temperature and prepare it for electron microscopy. We can call them all by a single word, embedding techniques.
Embedded tissue can be directly scanned and uploaded into digital form using electron microscopy, in a wide variety of ways. In recent years, we’ve been uploading ever larger amounts of resinated neural tissue into computer emulations, in increasingly automated ways, including whole animal brains. For example, a zebrafish larvae brain, about the size of the tip of a pencil lead, was recently imaged at the synapse level by a team at Rockefeller University in 2017. Teams at HHMI’s Janelia Campus and elsewhere imaged a whole fly brain in 2018. Both of these are about 100,000 neurons. Imaging a whole mouse brain’s connectome (80 million neurons!) was done by the Allen Brain Institute in 2019. Synaptome-level imaging of a whole mouse brain will eventually occur, and be an amazing advance.
Scientists can’t yet read complex memories from these fixed and scanned brains, but give them time. Many neuroscientists believe we need both higher fidelity in our tagging, scanning and emulations, so that they capture key features of the brains connectome 和 synaptome, both of which appear well-preserved by fixation, and better theories in computational neuroscience of how our dynamic, conscious brains interact with the static features of our brains. Those static features allow us to reliably retrieve our long-term memories over a lifetime, from special widely networked populations of neurons (so-called engram cells) even as our brains undergo constant chemical and structural changes, moment by moment.
Room-temperature preservation (RTP) via chemical fixation for whole human brains is exciting in several ways. First, it is potentially much cheaper than either traditional cryonics ($50-$80,000 in America today) or new cryonics (ASC) alike. I’ve seen estimates that noncryogenic fixation might be done for as low as $5,000 at scale, and $10,000 to start in America, assuming we can make it work for whole human brains. Right now, in urgent care settings adjacent to hospitals and hospices, if this process was validated, patients could be being fixed with glutaraldehyde over hours, and then transported over days to a few automated facilities, to have their brains fixed over several weeks (the process of separating a brain from a body is gruesome, but so are anatomical dissections, and both serve higher purposes). This low cost assumes that some of the professionals doing the urgent care and standby work would be volunteering their time (some retirees would find this to be very meaningful work) in a nonprofit. As popularity grew (if it ever did) all such work would surely become paid work. Legally, as with current cryonics, these bodies would be anatomical donations to these nonprofits. Second, RTP is conceptually much simpler than having to depend on an organization to maintain cryogenic temperatures. As there is much less for us to consider with respect to long-term care, that alone may make it a much more popular preservation choice.
I expect noncryogenically fixed brains, if they ever become a thing, will be stored in a great variety of ways, simply because people like options. Some will be stored in mausoleums or in the ground in progressive cemeteries. Some will be stored in safety deposit boxes in banks (giving them something truly valuable to protect, for once). Some will surely be taken home as family heirlooms, to be discussed with the children. Some will tour the country in scientific exhibits, as in Body Worlds today. Possessing the brains of our loved ones, and holding them in our hands, will help many of us to better cope with the loss and grief of death. Perhaps we’ll even pass a few laws requiring us to treat them with dignity, even if we don’t give them rights. They are anatomical donations, after all. The growing prevalence of such brains will give us new science-fictional stories about our potential afterlife, to complement the many valuable religious and cultural stories we already have. I gave a 45-minute talk, Chemical 脑部保存, at a futurist conference in 2012, exploring the positive potential of this idea for society.
What many people don’t know is that one of the teams competing for our small mammal brain preservation prize from 2012-2016 used noncryogenic fixation. This team from Heidelberg, Germany, which was led by Shawn Mikula, PhD, worked with a mouse brain. They used an even smaller protein crosslinker than glutaraldehyde, a chemical called acrolein, and osmium tetroxide to fix the fats. They used chemical diffusion (putting the brain in a bath of fixation chemicals) rather than the more difficult but more useful perfusion (putting the chemicals into the circulatory system) because the latter requires developing sophisticated protocols to make it work without blocking the circulatory system during fixation. As a result, their brain had a few areas that weren’t well preserved, and more importantly, it developed cracks as the resin dried. Mikula’s team might have solved the diffusion problem but they were not able solve the cracking problem with the resources they had, and we could not give any kind of award to cracked brains the way our prize rules were written.
That outcome was a minor tragedy, in my view, because if you think about it, cracks should not present a problem to automated scanning and uploading. Good software should be able to stitch together cracked brains in a digital upload, right? With adequate funding and effort, it seems quite reasonable to me that protocols should be able to be developed that allow us to fix brains of any size, using perfusion, not diffusion. Our circulatory system comes within a few millimeters of every neuron in our brain. With the right procedures, we should be able to make chemical brain preservation work. Alternatively, there are simpler methods, involving cutting the brain into sections, and doing simple diffusion, resinating them, and then stitching the images together later, that should be able to work for brains of any size. There are also a dizzying variety of promising and poorly investigated methods, including freeze substitution, that allow us to end up with fixed brains stable at room temperature.
Do We Need a Room-Temperature Preservation (RTP) Prize?
I would love to see BPF offer a new prize, one specifically designed for brain fixation ending with room-temperature preservation. Our Aspirational Neuroscience Prize does helpfully include fixation research, but it also covers a wide variety of desirable advances. I think we need a prize that is focused on this worthy challenge. The prize might have different awards (stages), for demonstrations of long-term stability (a month, a year, five years) of various key features of our connectome and synaptome, and it should focus on dependability and affordability. It might include awards for new methods like CLARITY, which remove lipids as plastination does, but then place the protein structure of the brain in a transparent acrylamide scaffolding, letting us take detailed pictures of neural microanatomy, and do repeated antibody- and gene-based labeling. Do “clarified” animal brains retain memories that might be read out in the future? It would be helpful to know. Such a prize should be written to encourage breakthroughs in stitching together separate sections and cracks, and provide awards even for imperfect preservation. For example, slicing a glutaradehyde-preserved brain into many sections, then diffusion resinating each section might prove the most reliable, inexpensive, and automatable approach to whole brain fixation. Can we make a good case that future computers should be able to stitch those sections together? Can our current stitching software be 95% accurate for small sections of neural tissue? If I and my family woke up as uploads in the future, and we were only 95% ourselves, or if only 90% of our memories made it to the future, I expect we’d all still much prefer that outcome over the alternative. Progress is always imperfect, and we each make our own calls on what uncertainty and risk we can accept.
Competing for an RTP prize would involve serious technical challenges and would not be easy or cheap. Fortunately, it involves challenges that many neuroscience labs will to have solve eventually in coming years, so a well-designed prize could accelerate that work. Progress in fixation and emulation might induce some current cryonics organizations to offer fixation in addition their existing options, and it could spur the creation of many new preservation startups. A generation hence, I’d love to see brain preservation services being offered by faith-based and secular groups of all types. The simplicity and affordability of fixation might even be one of the pushes needed to get brain preservation seriously discussed and debated by members of the neuroscience and medical ethics communities. After all, the more viable and affordable brain preservation options there are, the more the topic begins to demand public attention. For the last several years, both the traditional neuroscience and medical communities have been ignoring the brain preservation topic entirely, as evidence continues to mount that this technology is likely to preserve memories, at least, and thus should be evaluated and regulated. That neglect can’t continue forever.
Some of us find it interesting to argue whether a future upload would actually “be” us (that’s called the Copy Problem), and whether we would be likely to come back as self-conscious entities inside digital systems (that’s called the Qualia Question) or whether it is more likely that just our long-term memories (just the ones we wanted shared, of course) would be available to future family and society in an upload. In such discussions many of us come to realize that we actually are simulations already. All the parts of us that we care most about are special additions to our biology, built carefully over many years, via the formation of trillions of unique neural connections inside our heads. Anyone who has raised a child sees those new connections forming every day. If those same unique neural connections can be scanned and uploaded into artificial neural networks, with reasonably high fidelity, many of us would expect that our memories, at least, and perhaps our self-awareness as well, could be preserved for the future. Many of us can also imagine that giving our memories to the future, even if we personally didn’t come back, would be a good thing for society. Read Michael Malone’s The Guardian of All Things: The Epic Story of Human Memory, 2012, if you aren’t convinced on that point. Memory donation may eventually prove as beneficial and wisdom-generating for society as was the invention of language, and later, of writing.
What I do know is that if I and my family were facing an end-of-life choice today, we would not do traditional cryonics. We think it is too dependent on the preservation organization and too expensive for the average person in America and other developed countries, not even including emerging nations, which also deserve this choice. We don’t want to do anything that couldn’t be done by the average person in our society, as a matter of social justice. Noncryogenic fixation, by contrast, has the potential to be inexpensive and adopted enough that we might eventually get our health care systems to pay for it, as an end-of-life option for all who would want it. We spend much more than $10,000 on many end-of-life medical procedures at present, and I expect brain preservation will come to be seen as another reasonable and accepted choice in end-of-life care. We just need to get rich-enough and evidence-based enough as a society.
The bottom line is that some of us would choose to run the brain preservation experiment at the end of our lives today, and some of us wouldn’t, and we should all be fine with that diversity of outcomes. Unless science can tell us that the memory preservation goal is futile, I believe society has a duty to make the brain preservation choice as affordable and accessible as possible. How that actually happens is among the significant scientific, technical, political and medical questions all societies will have to face in coming decades.
What do you think? Would a Noncryogenic Brain Preservation Prize be something you’d like to see? What are the the drawbacks or benefits of this idea? Perhaps there is a philanthropist reading this who might be willing to fund such a prize. I’d love to hear your thoughts and feedback, either privately at johnsmart at gmail dot com, or shared publicly in the comments below. Thank you.









Wonderful essay, John. Thank you for writing it. I agree with you about many of the points.
One somewhat minor detail is that my impression of the plastination literature is that most researchers are generally not interested in microanatomy, so it is not clear how well the microanatomy is preserved.
One of the tricky things in studying the microanatomy of silicone plastinated tissue is doing so requires the use of sodium ions to cause depolymerization of silicone rubber, in a procedure known as deplastination.
One study evaluated pancreas and spleen tissue that had been fixed and then either plastinated or embedded in Epon (an epoxy resin) for EM [citation: 10.3109/10520299409106291]. This study found that ultrastructure was retained in pancreas and spleen tissue embedded in silicon rubber after initial fixation with a formaldehyde- and glutaraldehyde-containing fixative. They had difficulty with deplastination and re-embedding of pancreas tissue for ultrastructure studies, but deplastination was successfully performed on the spleen tissue.
The major variable in this study was the fixation method: the fixative that contained 0.56% glutaraldehyde in addition to 2% formaldehyde led to successful ultrastructural preservation regardless of the embedding procedure, whereas the fixative that contained formaldehyde alone did not lead to sufficient ultrastructure preservation. The authors concluded, “clearly, plastinated tissue retains its ultrastructure.”
Anyway, just a minor point that the microanatomy preservation of noncryogenic preservation methods, including plastination, is not very well studied.
Thanks for pointing this out Andy! I had no idea plastination could do that. As you point out, we know so little in this area still. I sincerely hope that changes sooner rather than later. Lots of folks today would like to have more affordable and accessible brain preservation options at the end of their lives, in my view.
I just discovered this article Nov. 27, 2020 after reanimation my interest in what Alcor and CI are up to lately. I dropped membership in Alcor in 2009 after having signed up in 1991 and following cryonics since 1979, having briefly been a member of CI in 1988 and TransTime in 1986. I was quite disturbed to discover and realize on some deep level that I had a limited lifespan at age 14 in 1972.
I found Ken Hayworth’s work and– Seung’s work- around 2009 as well, I think– I’d have to look at my notes. THIS article is a FOUNDATIONAL article for anyone like me interested in biological self preservation for later potential reanimation. I’ve widened out my possibilities to include astral travel, higher chakrik transcendance, reincarnation and Glorified Body as well as doing good works in the time I appear to have so that the EFFECT of my having lived might live on “forever”. Short of those things, I’m a documentarian with recordings of me on Philips audio tape as early as 1972 and plenty of photos and recently commentary of me going back to 1957. I expect to see other colleagues of mine in this area here soon as I help circulate your excellent post here. –Rick Potvin in Phoenix Arizona, bio-aged to an apparent 63–
There’s certainly a lot to find out about this subject. I love all of the points you made. Milicent Abel Aric
I think this is a great idea. What kind of funding would the noncryogenic preservation prize need? I’d be willing to pitch in a few hundred if it could be crowd funded.
I also did some more research on chemopreservation and found a well-researched wiki on the subject: https://github.com/ultrastructural-preservation/chemopreservation/wiki
Dear all, thanks kindly for your comments. Darin, thanks also for this question. I suspect (and others here at BPF may have different views) that the BPF would need a minimum of $100,000, likely from a small number of donors, to do meaningful targeted investigational work, using various perfusion protocols, and to sample and do circuit tracing on preserved small and large animal brains. I personally do not think crowdfunding would work at this stage, as I think we will need further progress in neuroscience before a significant number of people are willing to conditionally believe in the value of this work. Episodic memory retrieval from preserved model animal brains, beginning with small animals (flies, etc.) may be necessary to make the case for the value of this work, for humans. With luck, that may occur in the next few years. Progress in neuroscience and computational neuroscience is truly impressive these days. Warmest regards, JS
Yes, we also saw that lovely wiki, thank you. We are in communication with the author. We’d like to host a version of it on our site.
Digital scanning and uploading a consciousness into a computer won’t really be you, it’ll be a copy of you. It’s not a true transfer… we should focus on the ability to preserve and awaken people once we’ve achieved longevity escape velocity.
Hi JJ. Thanks for your comment. It gets to the true nature of identity, which is still a philosophical debate. At one level, it’s clear that the people who believe they will wake up in emulation will do so. They’ll have the same memories and believe they are the same person. At another level, it’s clear that we are dynamic patterns, being copied at the molecular level massively every second, just to keep our patterns stable. Digital emulation is just another form of copying—and if the emulation is complex enough—of living. Unfortunately I think your focus on “longevity escape velocity” is unlikely to yield more than another 20 or 30 years of healthy living for people. That is a worthy goal, to be sure, but no human alive knows how to repair postmitotic cells like the brain. Every model we have in nature for doing so, such as neoteny, would destroy the information in the brain in the process. In my view, only some advanced general AI, perhaps with the capacity to simulate human molecular biology at the quantum level, would have the capacity to redesign humans so that their neural information is “immortal.” It is possible that such redesign would have to incorporate digital technology at the level of the cell. We don’t have any examples in nature of differentiated postmitotic networks like neurons that can keep copying themselves indefinitely at the molecular level. So to my way of thinking at least, brain preservation is the only realistic path to continued growth and identity for all human beings who are dying today. I hope some of this resonates with you, and let me know if you think I am mistaken. Warm regards, John Smart